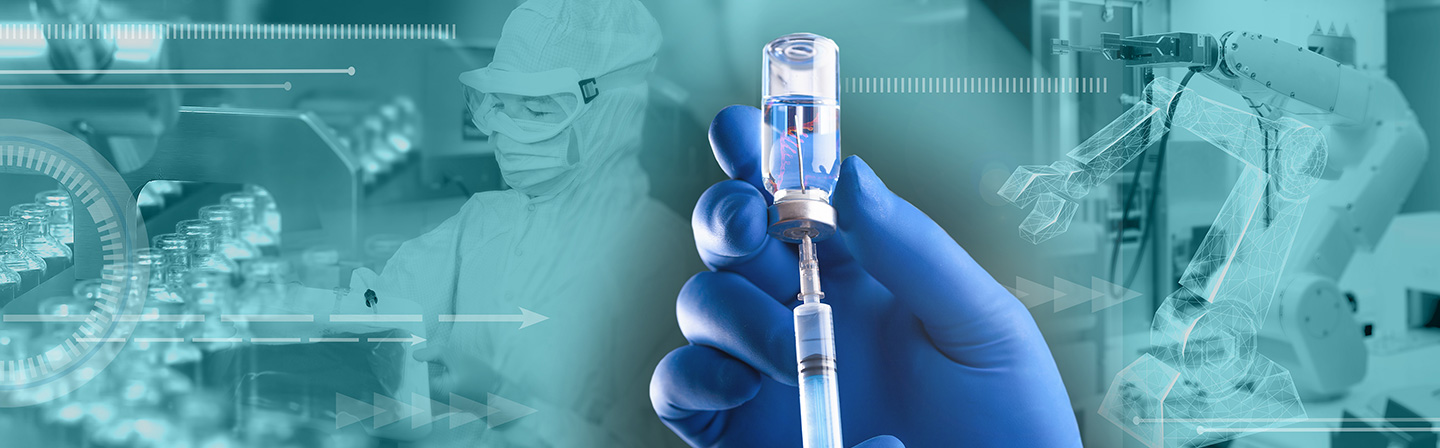
Flagship project

RNAuto – automated production of mRNA therapeutics
In the future, innovative pharmaceuticals such as new vaccines and mRNA-based gene and cell therapy methods will be available to large numbers of patients as part of an affordable healthcare system. This will create a need for automated production technologies that can safely and reliably produce drugs in accordance with the high standards required for pharmaceuticals (GMP certification). In order to develop an AI-driven, automated production process with digital monitoring capacities that is fit for the world of Industrie 4.0, the consortium is creating a pool of interdisciplinary expertise covering the fields of medicine, biology and engineering.
The project partners will concentrate on two drug candidates to demonstrate the process automation. The first is an mRNA vaccine for preventing West Nile fever, which is becoming endemic in Europe, while the second is an mRNA-induced gene therapy for treating cancer, based on natural killer (NK) cells from healthy donors.
The consortium is working on an automated screening system for rapid process development of mRNA nanotransporters, with a digital mapping function for process and quality control. They are also developing an expansion module with integrated quality control for manufacturing allogenic gene and cell therapies. The key biological challenges here are that the stability of mRNA molecules is fundamentally limited and that correctly encapsulating the mRNA in lipid nanotransporters is a difficult process. Producing mRNAbased pharmaceuticals in an industrially scalable way will be a key priority in the researchers’ work.
Lead management: Fraunhofer Institute for Cell Therapy and Immunology IZI
Further information
- https://s.fhg.de/RNAuto (izi.fraunhofer.de)
Participating Fraunhofer Institutes
- Fraunhofer Institute for Toxicology and Experimental Medicine ITEM (item.fraunhofer.de)
- Fraunhofer Institute for Microengineering and Microsystems IMM (imm.fraunhofer.de)
- Fraunhofer Institute for Manufacturing Engineering and Automation IPA (ipa.fraunhofer.de)
- Fraunhofer Institute for Production Technology IPT (ipt.fraunhofer.de)
- Fraunhofer Institute for Experimental Software Engineering IESE (iese.fraunhofer.de)
- Fraunhofer Institute for Microelectronic Circuits and Systems IMS (ims.fraunhofer.de)
- Fraunhofer Institute for Cell Therapy and Immunology IZI (izi.fraunhofer.de)